Reportable Events and Non-Compliance
Institutional Review Board ∙ irb@umd.edu ∙ 301-405-4212 ∙ 1204 Marie Mount Hall ∙ Hours: 8:30 AM - 4:30 PM
KUALI-IRB LAUNCH (1/5): Kuali-IRB is LIVE for new projects and transitions! For more information, see the Transition to Kuali-IRB page.
Adverse Events
Adverse events should be reported to the IRB within 72 hours of the research team learning of the event, by using the Adverse Event/Unanticipated Problem Report (IRBNet only) or the Reportable Events form in Kuali-IRB (guidance here).
An adverse event (AE) is defined as event that occurs during the course of a research project that either causes physical, social, economic, or psychological harm, or increases the risk of physical, social, economic, or psychological harm, or results in loss of privacy and/or confidentiality to a research participant or others (i.e. family members). Adverse events include expected and unexpected harmful effects and unexpected harms of an interaction or an intervention.
Harm is “unexpected” when its specificity and severity are not accurately reflected in the consent document. Harm is “related to the research procedures” if in the opinion of the principal investigator, it is more likely than not to be caused by the research procedures or if it is more likely than not the event affects the rights and welfare of current participants.
Examples of adverse events include:
- a breach in participant confidentiality;
- participant complaints;
- incarceration of a participant in a project not approved to enroll prisoners;
- injury and/or death of a research participant that is unrelated to participation in the research.
Serious adverse events (SAE) are those that (1) result in death, (2) are life-threatening, (3) require inpatient hospitalization or prolongation of existing hospitalization, (4) result in persistent or significant disability/incapacity, (5) result in a congenital anomaly/birth defect, or (6) are an important medical event that jeopardizes the subject or requires medical intervention to prevent one of outcomes listed above.
Unanticipated Problems
Unanticipated Problems (UP) should be reported to the IRB within 72 hours of the research team learning of the event, by using the Adverse Event/Unanticipated Problem Report (IRBNet only) or the Reportable Events form in Kuali-IRB (guidance here).
How to determine whether an Adverse Event is an Unanticipated Problem:
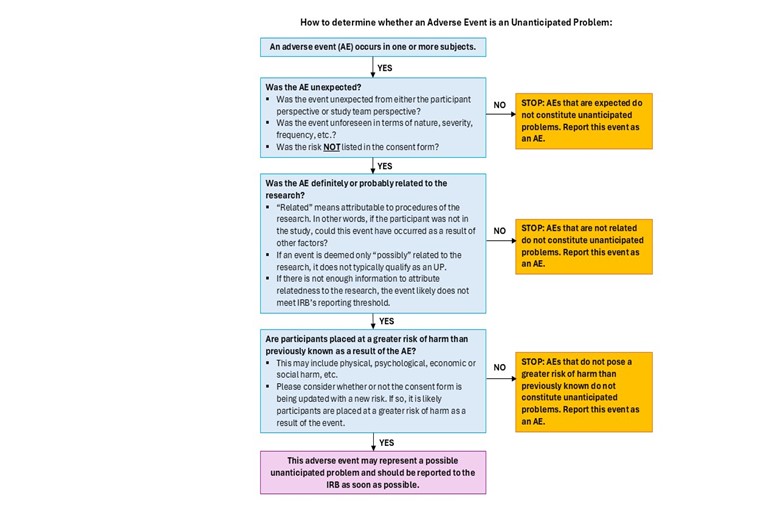
Unanticipated problems are defined as any incident, experience or outcome that meets ALL of the following criteria:
- Unexpected (unforeseen by the researcher or the research participant) in terms of nature, severity, or frequency, given the research procedures and the subject population being studied;
- Related or probably related to participation in the research, or if the event or problem probably or definitely affects the safety, rights and welfare of current participants; and
- Suggests that the research places subjects or others at a greater risk of harm (including physical, psychological, economic or social harm) than was previously known or recognized.
Deviations
Deviations should be reported to the IRB within 72 hours of the research team learning of the event, by using the Deviation Report (IRBNet only) or the Reportable Events form in Kuali-IRB (guidance here).
A deviation is defined by any difference in study conduct from the criteria or activities prescribed in the IRB approved project, which may or may not affect the participants' rights, safety, welfare, and/or the integrity of the study.
Examples of deviations include:
- Enrolling more than the approved number of participants for a research study;
- Changes to study procedures without prior IRB approval;
- The addition/implementation of new study materials without prior IRB approval.
Reporting Non-Compliance
The University is committed to an open and honest campus environment, and we ask you to support this commitment. This ensures UMD is following the highest standard of ethics. In situations in which you prefer to make a confidential or anonymous report, you are encouraged to use the UMD Compliance Reporting System. It is a safe way to report problems or raise concerns, and each report will be followed up appropriately.
To anonymously report a human subject research concern please visit the UMD online Ethics, Integrity and Compliance Reporting system.
Non-Compliance is defined as a failure to follow the regulations, Maryland state law, institutional policy, or the requirements or determinations of the IRB. Non-compliance may range from minor to serious; be unintentional or willful; and may occur once, sporadically, or continuously. The degree of non-compliance is evaluated on a case-by-case basis.
Continuing Non-Compliance includes multiple or repeated instances of non-compliance, particularly after written notice from the IRB that the investigator must take action to correct the non-compliance, or from the Institutional Official (IO) that the IRB or individuals within UMD must take action to correct non-compliance. Continuing non-compliance may occur on one or more than one study and may occur over a period of time.
Serious Non-Compliance involves non-compliance such that the failure to follow federal regulations, state laws or institutional policies relevant to human participants research or any determinations of the reviewing IRB and involves one or more the following: substantive harm or genuine risk of substantive harm to the safety, rights and welfare of research participants or others, decreases potential benefits, or compromises the integrity of the human research protection program.
Research Misconduct is defined as any fabrication, falsification, or plagiarism in proposing, performing, or reviewing research or reporting research results. This definition derives from federal regulation and is reflected in the University of Maryland Policy and Procedures Concerning Scholarly Misconduct, which governs the review of allegations of research misconduct at the institution. Instances meeting the definition of research misconduct will be reported to the Research Integrity Officer (RIO) in the Office of Faculty Affairs (OFA) by the Director of the HRPP, the IRB Chair, or IRB Vice-Chair.
University System of Maryland Reporting Resources:
Employees, students and other members of the University System of Maryland (USM) community are strongly encouraged to communicate suspected fraud or other financial irregularities to appropriate authorities.
At the Report Fraud, Waste, and Abuse website and via telephone "Hotline" (1-877-330-2320), members of the USM community may report such concerns. This user-friendly process is both anonymous and confidential. It is designed to deter and detect fraud, unethical business practices, and financial irregularities at USM institutions.
In addition to the portal and hotline, reports of fraud may be submitted to a fax number and mailing address. A full summary of contact information for all of these channels follows at the bottom.
We encourage anyone familiar with any fraud, misuse or misappropriation of USM resources to use the Web portal, hotline and other channels to confidentially and anonymously report suspected fraud so that it may be appropriately investigated.
For more information visit the USM Communication Channels for Anonymous, Confidential Reporting of Fraud page.
Mandated Reporting
As per State law, all researchers must report all suspicions of current or past incidents of child abuse or neglect to the Chief of Police and submit a written report to Child Protective Services within 48 hours, as per University of Maryland, College Park’s policy. Regardless of whether the participant is currently an adult, the researchers are responsible to report cases of suspected child abuse or neglect that are presently occurring or occurred in the past.
An abbreviated list of Frequently Asked Questions when Reporting Child Abuse and Neglect in the University System of Maryland can be found here. For a complete list, please visit the University of Maryland, College Park’s policy.
