IACUC Protocol Submission
What kinds of activities require an Animal Study Protocol, and who must submit?
Activities at UMD that involve animals in research, training, or testing must be reviewed and approved by the IACUC prior to ordering animals for the purposes of the activity and before beginning any work involving animals. Specifically, Animal Study Protocols must be submitted for the following:
Faculty Research - All animal research activities performed at UMD must be conducted under an IACUC-approved Animal Study Protocol. Most principal investigators (PIs) are faculty (tenured/tenure track or professional track), with a few exceptions.
Student research - Animal research activities carried out by students must also be conducted under an approved IACUC protocol, which must be submitted by their faculty advisor. This includes research performed by graduate or undergraduate students, or students working as part of a research team (e.g., GEMSTONE). In all cases, the faculty advisor must sign off on the research protocol submitted to the IACUC.
If the student will perform animal research at an outside (PHS-assured) institution, the protocol may be submitted to and approved by that institution’s IACUC; however, a copy of the protocol, the protocol approval (if separate from the protocol document), and any external funding information must be submitted to the UMD IACUC. For student research at non-PHS assured institutions/agencies/facilities, see Non-assured Performance Sites, below.
Non-assured Performance Sites
Any awardee (faculty member or student) who is planning to perform research funded by the PHS at an outside institution/agency/facility that does not have it’s own PHS assurance must ensure that the outside institution either negotiates its own PHS Assurance or becomes covered in the UMD PHS assurance. This places the un-assured institution under the UMD PHS assurance, and UMD must then treat it the same as all other facilities included in their program (perform semi-annual inspections, include in reports to OLAW, etc.).
Teaching - Courses taught at UMD that involve the use of animals must also be covered under an IACUC-approved protocol, which should be submitted by the faculty member in charge of the course.
Field Studies - An IACUC-approved protocol is required for any study that involves the capture, handling, or other direct manipulation of animals in the wild. If animals will not be handled or manipulated, but only observed, a research protocol may not be required. It is recommended that any PI planning observational-only field studies contact the IACUC office to confirm whether or not they need to submit a protocol.
External Collaborations - PIs participating in external collaborations at PHS-assured institutions, where the animal research has been submitted to and approved by the outside institution’s IACUC, do not need to submit a full protocol to the UMD IACUC. However, the UMD PI must submit a copy of the protocol, the protocol approval (if separate from the protocol), and a copy of any funding proposal in support of the work.
Agricultural Extension Activities - Animal teaching/demonstration activities conducted either with UMD-owned animals or at UMD facilities must be reviewed and approved by the IACUC.
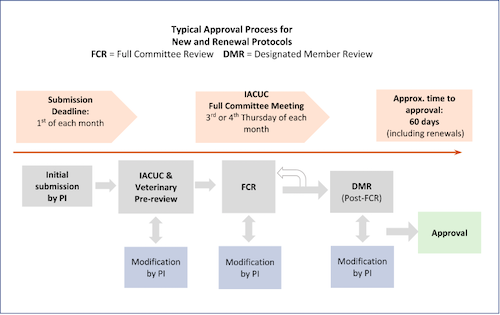
All new animal research protocols, renewals, and amendments to protocols (with a couple of exceptions) must be reviewed by one of two equally acceptable methods: Full Committee Review (FCR), and Designated Member Review (DMR). There is no “expedited review” option for items submitted for review by the IACUC, however it is generally true that the DMR process takes somewhat less time than FCR. Below is a flow diagram depicting the process of approval for items sent to FCR, with approximate processing times. Note: each submission is different, and many factors influence time to approval, so individual processing times will vary. Response time by the PI is often a significant contributor to overall time to approval.
Full Committee Review
Typically, new protocols, protocol renewals, and complex amendments are sent for FCR. These must be submitted by the first of each month to be considered by the IACUC at the monthly meeting. Below is a diagrammatic representation of the FCR process, with deadlines and approximate time to approval.
Designated Member Review
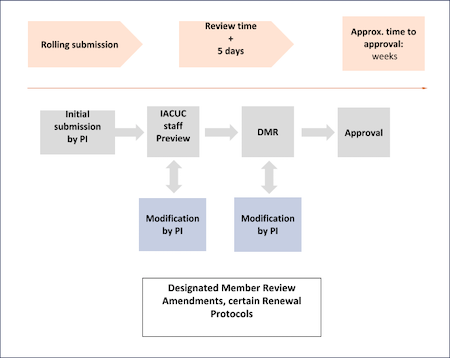
Amendments are typically, but not always, sent out for DMR (check with the IACUC office if you are unsure). All DMR items are assigned to at least two members of the committee for review, and are shared with the entire committee on IRBnet. Per our assurance In general, members are given 3 calendar days to call the item to FCR, if they so choose. (A committee member may call any protocol or amendment to full committee review at any time.) Once the callback date has passed and the reviewers have submitted comments, the PI will be notified regarding any requests for modifications, see diagram below.
Below are several “How To” documents detailing how to navigate the IRBnet system, listed on IRBNet under “Forms and Templates.” If these do not answer any of your questions, please feel free to contact the IACUC office.
- FAQ's (PDF)
- Did I...? Commonly Overlooked Submission Details (PDF)
- How to Create a New Protocol (PDF)
- How to Share a new Protocol (PDF)
- How to Submit a New IACUC Protocol (PDF)
- How to Submit an Amendment (PDF)
- How to Submit an Annual Review (PDF)
- How to Clone an ASP Form (PDF)
- How to Document External Collaborations (PDF)
- How to Return to Edit Your Project (Pre-Submission) (PDF)
From: Animal Care Resource Guide Policies USDA, AC (2011)
“The Animal Welfare Act (AWA) regulations require principal investigators to consider alternatives to procedures that may cause more than momentary or slight pain or distress to the animals and provide a written narrative of the methods used and sources consulted to determine the availability of alternatives, including refinements, reductions, and replacements.”
The research study protocol requires justification of certain items including animal species and number, use of non-pharmaceutical grade reagents, and others. A very specific requirement is placed on the justification for any pain or distress experienced as a part of the research design. This page is aimed at providing guidance on how to perform the search for alternatives to procedures that may cause pain or distress.
ALTERNATIVES TO ANIMAL USE - IACUC POLICY
The IACUC advocates the use of animal alternatives as described by the 3 Rs, specifically
- REPLACEMENT of animals when possible,
- REDUCTION of in the animal numbers used, and
- REFINEMENT of the animal procedures to minimize discomfort as much as possible
We also believe in a fourth R -- RESPONSIBILITY
“How to” sites for alternative approaches:
Center for Alternatives to Animal Testing (Johns Hopkins University)
Discussions of the 3Rs, refinement for rats and mice, information on alternative searches, links to databases, and specific search templates.
Fund for the Replacements of Animals in Medical Experiments (FRAME)
Guides for conducting alternative searches including information on search terms and strategies as well as links to databases.
Alternative Methodologies (Animal Welfare Information Center)
Guides for conducting alternative searches including information on search terms and strategies as well as links to databases.
Databases for conducting alternative searches:
Alternatives to Animal Testing on the Web - AltWeb (Johns Hopkins School of Public Health)
Information on alternative searches AND a search engine specifically designed to search for alternatives to animal procedures.
Animal Welfare Information Centers (USDA National Agricultural Library)
Established under the 1985 amendment to the Animal Welfare Act, this office provides information on how to do alternative searches as well as access to various databases (e.g., Agricola). The office may also be contacted to perform an alternative search for you.
Other informational Resources
Norwegian Reference Center for Laboratory Animal Science & Alternatives - NORINA
Information on alternatives to animal use in teaching and research methodologies.
ECVAM: European Centre for the Validation of Alternative Methods
Provides access to the SIS - Scientific Information Service - databases on advanced alternative methods to animal experiments in biomedical sciences.
There are several items that should be included in your submission, along with the ASP and section forms. Frequently, these items are inadvertently omitted, which can slow down the approval process. Please see below for a list of those items that must be included for a protocol to be approved.
Personnel Qualifications Form
All personnel performing research, training or testing activities with animals must be named as key personnel on the research protocol. A Personnel Qualifications Form (PQF) for each individual listed on the protocol must be submitted with the initial protocol submission and/or with subsequent personnel amendments. This form indicates the skills and expertise the individual has or needs training for, in order to perform work on the protocol. Dates of training completion and Occupational Health Program enrollment must be provided on the PQF (see below). The PQF can be found on IRBnet, under Forms and Templates. When adding personnel to an existing protocol, the online ASP form must be updated to reflect the new personnel working on the project.
Animal Users Training documentation
Prior to approval of either new protocols or personnel additions to existing protocols, all key personnel must complete the PI/Animal Users Training course, which provides users with a comprehensive understanding of the laws, regulations, local policies and procedures associated with performing research with animals. All personnel must take refresher training every 3 years.
Occupational Health Program Enrollment
All personnel working on animal research must submit the Animal Handler’s Risk Assessment form to the University Health Center Occupational Health unit (See also “Occupational Health”). Specific questions regarding occupational health may be addressed to the University Health Center, or the Occupational Health Physician.
ESSR approvals and training documentation for the use of hazards
At the time of submission, new protocols must include approved ESSR registrations for the use of hazards (e.g., biologicals, radiation) and any associated training (e.g., BSL2, BSL3). The same documents should be submitted for any amendment adding new regulated hazards.
Signatures
All submissions must be signed by the PI, and new protocols must be signed by the department chair and the facility manager. These signatures ensure that the appropriate resources (housing, husbandry staff, etc.,) are available for the animals that will be housed under the protocol. These signatures are required for protocol approval.
Protocol Review Worksheets/Checklists and Regulatory Source Language
The Protocol Review Worksheet is a tool to help the IACUC committee review protocols. PIs can use this worksheet to help ensure that their protocol package is complete. The Regulatory Source language Worksheet shows which federal policies, regulations, and guidelines the IACUC uses during protocol review.
The UMD Department of Environmental Safety, Sustainability and Risk’s webpage provides information and training opportunities for Biological, Chemical, Radiation, Field Studies, Research Diving and other risk areas.
To request a text-only version of any PDF on this page, please contact the IACUC.
