A collaboration between faculty researchers in the College of Computer, Mathematical, and Natural Sciences (CMNS), the A. James Clark School of Engineering (ENGR), and the First-Year Innovation and Research Experience (FIRE) program supported by the University of Maryland Grand Challenges Grants program is focused on devising innovative solutions for helping our bodies clear respiratory infections.
Mucus plays an important role as part of our barrier immune response. Depending on the molecular make-up of the mucus, it can either protect us from respiratory bacteria and viruses, or result in thicker mucus that is difficult for our lungs to clear. This build-up of mucus is seen in numerous diseases (e.g., cystic fibrosis, COPD, COVID-19) and can lead to chronic infections.
Mucus is made up of different mucin proteins, inorganic salts and water. Mucins are large heavily glycosylated proteins that result in a gel-like network that can serve as a net and barrier to protect our cells from microbes. However, the molecular nature of mucus can shift over the course of a respiratory infection and in chronic respiratory diseases. Of the secreted mucins in the hydrogel layer, increases in MUC5AC (relative to MUC5B) result in thicker, more viscous mucus which is associated with poorer outcomes in respiratory diseases.
Because MUC5AC is overexpressed in disease conditions, the team's goal is to engineer a protease that can recognize and specifically cleave MUC5AC, but leave the other mucins untouched. The research team includes Associate Professor of Cell Biology and Molecular Genetics Louisa Wu (CMNS), Associate Professor of Bioengineering Gregg Duncan (ENGR), Associate Clinical Professor in the FIRE Program Catherine Spirito, Professor Emeritus of the Institute for Bioscience and Biotechnology Research Philip Bryan, and Clinical Assistant Professor in the FIRE Program Quira Zeidan.
The first pass protease (pT2326) is designed to cleave the sequence DTFQNL-RD (122-131) found in MUC5AC and not MUC5B. This protease does not yet incorporate an aptamer localization element or additional regulatory components, 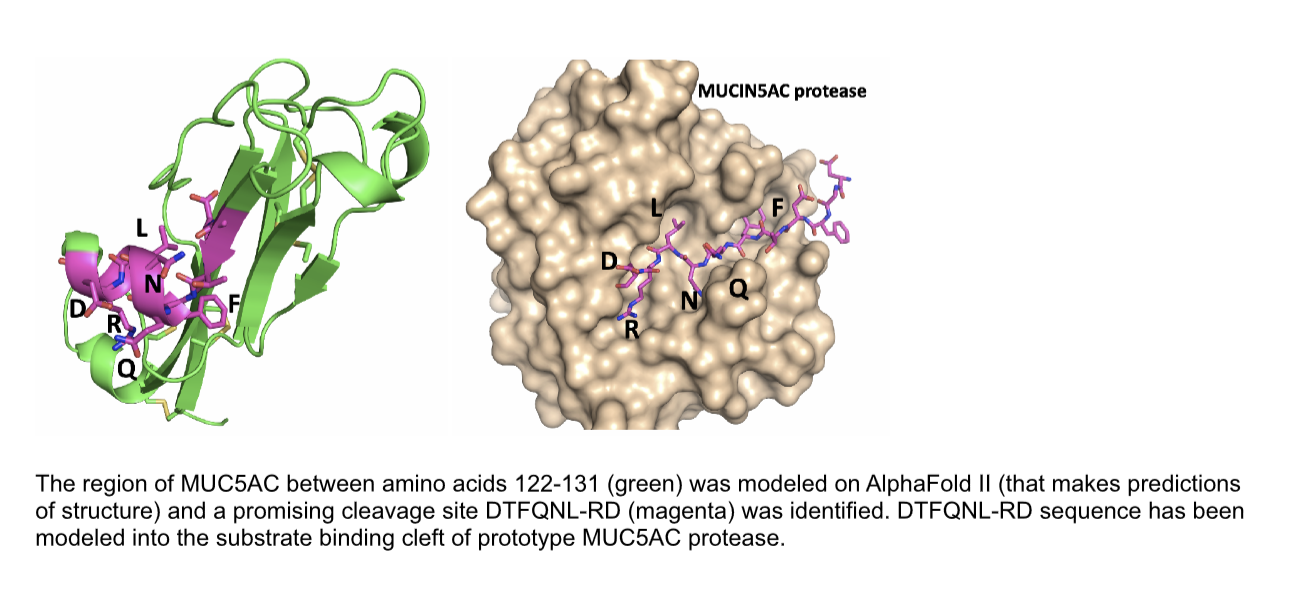 which will be added later to enhance specificity and efficacy. The protease has been tested for its effect on the viscosity of a mucin gel harvested from airway epithelial cells. Of note, the protease results in a significant increase of movement of a fluorescent particle in the mucus gel layer, indicating that the viscosity of the gel has decreased. A greater proportion of MUC5AC in a mucus gel layer is known to contribute more to viscosity, hence this result suggests that the protease may be acting on its desired target. Cell biology and biochemical experiments to test this are currently in progress.
which will be added later to enhance specificity and efficacy. The protease has been tested for its effect on the viscosity of a mucin gel harvested from airway epithelial cells. Of note, the protease results in a significant increase of movement of a fluorescent particle in the mucus gel layer, indicating that the viscosity of the gel has decreased. A greater proportion of MUC5AC in a mucus gel layer is known to contribute more to viscosity, hence this result suggests that the protease may be acting on its desired target. Cell biology and biochemical experiments to test this are currently in progress.
The use of a designer MUC5AC protease to specifically localize and cleave the mucin protein that builds up under pathological conditions, but leaves the mucins associated with healthy conditions alone, would be the first of its kind. A therapeutic with this sort of molecular precision is ideal in that it could successfully break down the more viscous mucus seen with disease, but leave healthy mucus alone.
To learn more about this project, visit: https://research.umd.edu/respiratory.
